Research led by Sichuan University and Huazhong University of Science and Technology, China, has revealed genetic mechanisms that could prolong healthy aging. In the paper, titled “Partial inhibition of class III PI3K VPS-34 ameliorates motor aging and prolongs health span,” published in PLOS Biology, the team details the methods they used to narrow down the potential genomic pathways to a single gene that could be critical to extending healthy human longevity.
With a combination of genetic manipulation, behavioral assays, microscopy techniques, and electrophysiology, the researchers investigated the role of VPS-34 in motor aging. These methods allowed the researchers to gain insights into the molecular mechanisms underlying motor aging and the effects of VPS-34 inhibition on motor function, synaptic transmission, and muscle integrity.
According to the authors, increased life expectancy in recent decades has not been accompanied by a corresponding increase in health span. Aging is characterized by the decline of multiple organs and tissues and motor aging, in particular, leads to frailty, loss of motor independence, and other age-related issues. Identifying mechanisms for therapeutics to delay motor aging is crucial for promoting healthy aging.
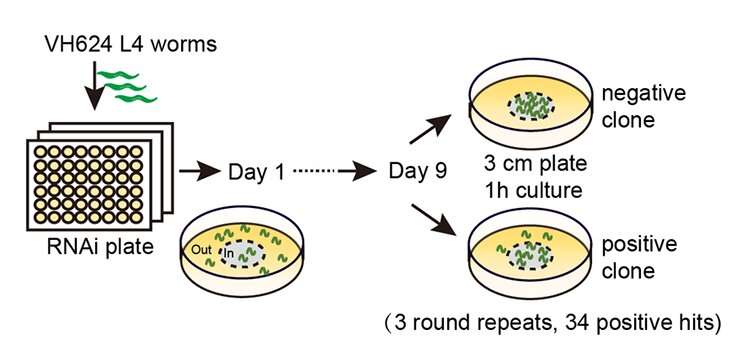
The C. elegans worm is a widely used model for aging studies, and previous research has explored candidate genes involved in motor aging in both worms and mice. Despite previous research efforts, there is still a lack of knowledge of the evolutionarily conserved mechanisms that regulate motor aging. The team developed high-throughput screening methods to identify potential regulators of motor aging.
To begin, the researchers tested worms for good or poor late-life fitness. Worms were placed in a 1-cm-diameter circle on a culture plate, and after one hour, the percentage of worms that moved outside the circle (out-of-circle ratio) was quantified. To assess motor activity decline during aging, the researchers monitored the worms from young adulthood (day 1) to old age (day 9), measuring the out-of-circle ratio every other day.
Researchers compiled a list of likely gene targets by comparing worm gene expression through a genome-wide RNA interference (RNAi) screen. Through multiple rounds of screening, they identified several candidate genes that consistently increased motor activity when knocked down.
Among the top hits, they focused on the gene VPS-34 (human homolog PIK3C3) and found that its partial inhibition significantly improved neuromuscular synaptic transmission and muscle integrity in both worms and mice. Mice treated with a VPS-34 inhibitor performed significantly better in the treadmill running assay.
The authors suggest that VPS-34 is an evolutionarily conserved regulator of motor aging and provides a potential actionable target for delaying motor aging and prolonging health span. Partial inhibition of VPS-34 could be a viable treatment strategy to improve motor function during aging. The study also highlights the importance of cell type-specific mechanisms, as VPS-34 primarily functions in motor neurons to regulate motor aging.
Future studies are needed to explore the functional results after VPS-34 inhibition to better understand the benefits and deleterious effects of any potential treatment strategy in the brain and other organs and cell types.
For example, VPS-34 is known to be involved in a wide variety of cellular tasks, including autophagy, allowing cells to recycle old or damaged parts and repair themselves. Long-term inhibition could potentially lead to adverse effects on organismal health and viability.
VPS-34 is conserved among all eukaryotes, and while indications of improved function may have been observed in the study scenario, further research is needed to fully understand the implications and potential risks associated with VPS-34 inhibition in different contexts.